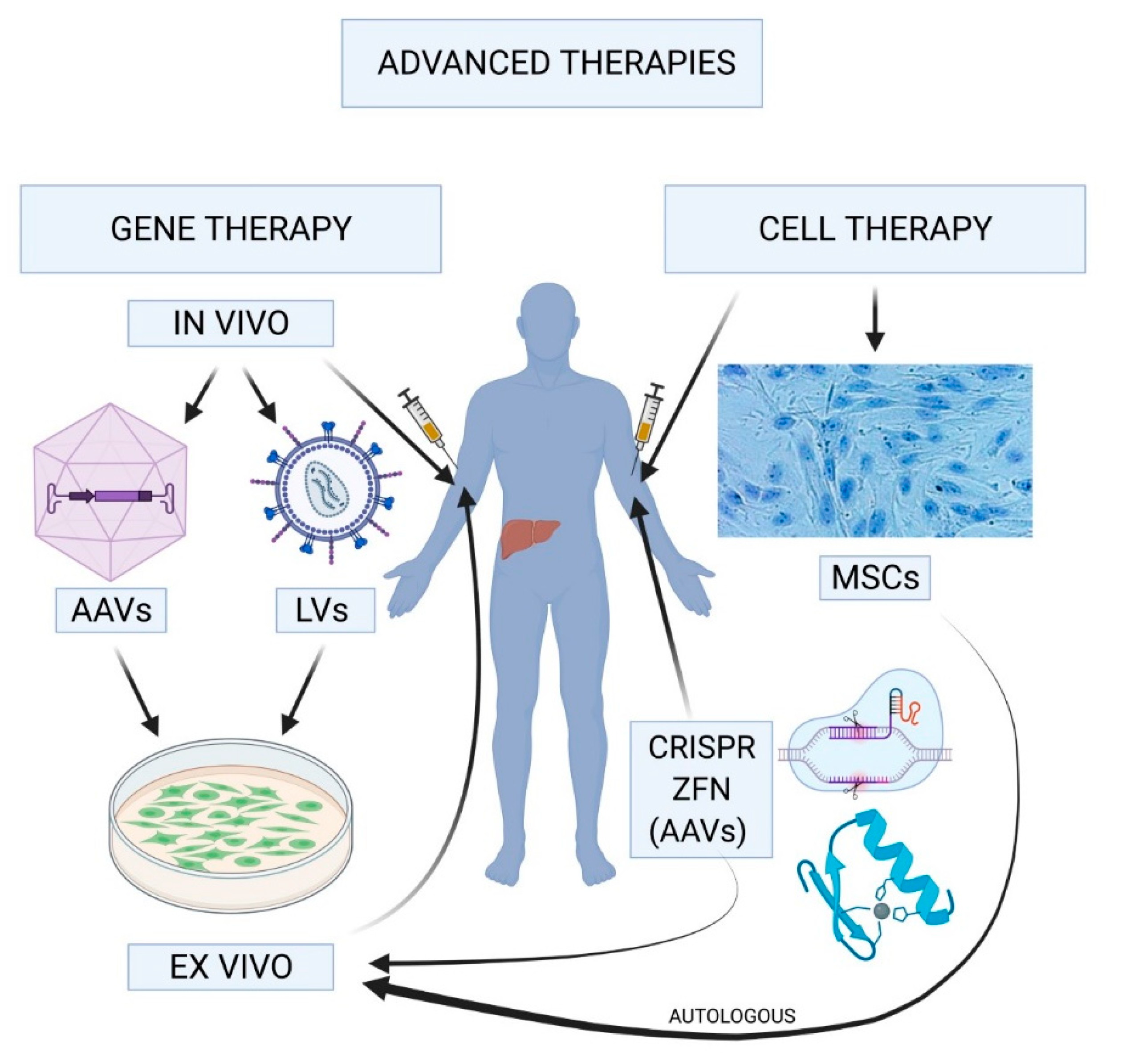
Hemophilia gene therapy is revolutionizing treatment options for individuals affected by this challenging condition. Once bound by the limitations and dangers of traditional therapies, patients now have a glimmer of hope with innovations like Hemgenix, a groundbreaking treatment that targets the underlying genetic causes of hemophilia B. With its recent FDA approval, this therapy represents a significant leap in the impact of gene therapy on the healthcare landscape, promising not only to alleviate the symptoms but also to provide lasting solutions. News surrounding hemophilia B is increasingly focused on these advances in gene therapy, offering patients a chance to improve their quality of life while reducing the burden of living with hemophilia. As more individuals explore the potential benefits of gene therapy for hemophilia, the medical community is on the verge of redefining the future for those coping with this condition.
The advent of innovative treatments for hemophilia has paved the way for alternative approaches that promise transformative outcomes. Often referred to as genetic modification therapies or advanced forms of cellular intervention, such techniques aim to correct the mutations responsible for hemophilia, allowing patients a reprieve from the constant monitoring and treatment regimens that have historically defined their lives. With options like Hemgenix leading the charge, the landscape of hemophilia management is changing, offering hope for a more carefree existence without the constant preoccupation with potential bleeding episodes. This shift is not just about medical advancements; it’s about enhancing the lives of those who live with hemophilia by reducing their treatment demands and bringing us closer to a future where this condition may no longer dictate their daily activities.
Understanding Hemophilia and Its Challenges
Hemophilia is a genetic disorder that impairs the body’s ability to control bleeding. Individuals living with hemophilia often experience spontaneous bleeds which can be internal and are unpredictable in nature. This chronic condition primarily affects males, as hemophilia is linked to the X chromosome. The CDC estimates that about 33,000 American males are living with hemophilia, facing unique challenges that involve regular medical visits, needle injections, and constant vigilance to prevent injury. From a young age, many with hemophilia, like Terence Blue, learn the importance of managing their condition, which often means living with the fear of bleeding episodes.
As the management of hemophilia evolved with medical advancements over the decades, patients have benefited from synthetic clotting factors that significantly reduced the risks previously associated with treatments. These innovations have contributed to extending life expectancy for hemophilia patients, bringing it closer to the average male population. However, despite these advancements, emotional and social challenges remain prevalent. The stigma of explaining the condition to peers and needing to avoid certain activities due to the risk of injury can exacerbate feelings of isolation and anxiety. Thus, while the physical care has improved substantially, the daily lifestyle adjustments and the mental burden associated with hemophilia remain significant.
The Groundbreaking Gene Therapy for Hemophilia
The emergence of gene therapy represents a beacon of hope for individuals living with hemophilia. In recent years, advances such as Hemgenix have shown promise in treating hemophilia, particularly hemophilia B. This innovative therapy works by leveraging a modified virus to deliver a healthy copy of the gene responsible for producing clotting factors directly into the patient’s liver, ultimately enabling them to produce their own clotting factor IX. This paradigm shift signifies a move from regular injections of synthetic factors to a potential one-time solution that addresses the underlying genetic cause of the disease.
Clinical trials have demonstrated that these gene therapies can lead to sustained increases in factor levels, significantly reducing or eliminating the need for traditional prophylactic treatments. For instance, 94% of patients treated with Hemgenix in clinical studies did not require factor IX prophylaxis three years later. This substantial efficacy not only represents a medical breakthrough but also leads to improved quality of life for patients, who can enjoy a more normalized lifestyle without the constant worry of administering injections or experiencing severe bleeds.
Impact of Gene Therapy on Real Lives
The impact of gene therapy extends beyond just a clinical outcome; it deeply affects the personal lives of those diagnosed with hemophilia. Terence Blue’s journey illustrates this firsthand. After receiving Hemgenix, he experienced an impressive turnaround in his body’s healing capabilities, marking a departure from his past experiences with bleeding. Stories like Blue’s show how gene therapies can drastically improve life for individuals who have been managing a chronic condition for decades, alleviating fears that have colored their daily existence.
The potential of these therapies to transform lives has ignited professional and public interest alike. As more patients like Blue embrace these treatments, the narrative surrounding hemophilia is changing. Individuals who previously felt chained to their condition can now envision a future with less dependence on frequent medical treatment and a decreased fear of injury. This newfound freedom encourages patients not only to live fuller lives but also to consider activities and opportunities that they may have previously avoided due to their hemophilia.
Navigating the Market Landscape of Gene Therapies
Despite the excitement surrounding gene therapy for hemophilia, the landscape is also fraught with challenges relating to market dynamics. With the development and approval of therapies like Hemgenix, pharmaceutical companies must navigate high costs of research and development coupled with market pressures that influence patient access. The price of hemophilia gene therapies such as Hemgenix, with an estimated cost of $3.5 million, raises questions about sustainability and accessibility. As seen with other recent gene therapies, if the cost is deemed prohibitive, even the most promising treatments risk being withdrawn from the market.
Additionally, patient acceptance plays a critical role in the adoption of these therapies. The reluctance of some patients to switch from traditional treatment regimens to gene therapy often stems from concerns regarding efficacy, side effects, and the novelty of the approach. It is essential for healthcare providers to educate patients about the transformative potential of gene therapies, as well as address their valid worries, to facilitate informed decision-making and improve the overall acceptance and uptake of these groundbreaking treatments.
The Future of Living with Hemophilia
As advancements in gene therapy continue, the future of living with hemophilia looks increasingly promising. The ongoing development of innovative therapies that tackle the root causes of hemophilia heralds a shift from chronic management to possible eradication of symptoms. The experiences of patients like Terence Blue serve as testimonials to the potential of these treatments, showing that effective, permanent solutions might soon become a reality for many individuals affected by hemophilia B and other genetic disorders.
Research and clinical innovations also reflect a broader trend in medicine, where gene therapies are expected to proliferate for various conditions, not just hemophilia. With ongoing regulatory support and burgeoning technological progress, the horizon is bright for patients, paving the way for a future where life expectancy and quality of life for those with hemophilia are significantly enhanced. As the evolving landscape of gene therapy expands, there is every reason to believe that the goal of a manageable, if not entirely cured condition, will be achievable for future generations of hemophilia patients.
Understanding Hemgenix: Mechanism and Approval
Hemgenix, a groundbreaking therapy, gained FDA approval in late 2022, offering a revolutionary approach for hemophilia B treatment. The mechanism of action involves using a specially modified virus to deliver genetic material into the liver, which then enables the body to produce its own clotting factor IX. This innovative strategy minimizes the risks associated with blood transfusions and ongoing daily treatments traditionally used in managing hemophilia. The swift advancement from laboratory research to clinical application exemplifies how dedicated scientific efforts are translating into meaningful therapies that can dramatically change patients’ lives.
The approval of Hemgenix has spurred optimism within the medical community, laying the groundwork for more research into gene therapies across a spectrum of genetic conditions. The commitment to supporting ongoing trials and potential new treatments is crucial. As more patients undergo therapies like Hemgenix and benefit from increased clotting factors, there is a compelling narrative for further investment in gene therapy research, aiming to uncover solutions for hemophilia and beyond. This evolving landscape is rooted in hope and innovation, heralding a new era in the treatment of genetic disorders.
Market Challenges: The High Cost of Gene Therapies
One prominent challenge facing the distribution of gene therapies such as Hemgenix is the exorbitant cost associated with their development and administration. The single-dose approach, while incredibly beneficial, presents an economic conundrum as it requires substantial investment without the promise of recurrent patient reimbursements seen in chronic disease therapies. The high price tag can deter insurers from covering treatments, resulting in limited access for patients who may benefit from these breakthrough innovations.
Moreover, drug companies must balance the financial viability of their treatments with the needs and expectations of patients. The withdrawal of therapies from the market, as has been seen with some hemophilia treatments, emphasizes the need for ongoing dialogue between pharmaceutical companies, healthcare providers, and patients to foster an environment where innovative treatments can be both effective and sustainably priced. Only through addressing these financial barriers can we ensure that groundbreaking therapies like Hemgenix are available to those who need them most.
Living with Hemophilia in Modern Times
For many people living with hemophilia, the daily reality is a careful balance of managing symptoms and pursuing a normal life. Thanks to advancements in treatment like Hemgenix, patients can hope for an improved quality of life that only a few years ago seemed improbable. The combination of effective therapies and community support enables individuals with hemophilia to engage in social activities and explore new interests without the same fears of bleeding that have historically limited their experiences.
Support groups and educational initiatives play a vital role in helping families understand hemophilia and navigate the complexities of living with the disorder. Increased awareness and acceptance within society contribute to alleviating the stigma associated with bleeding disorders, fostering an environment where individuals are empowered to share their experiences and advocate for their health needs. This shift in perspective encourages a culture of openness, self-advocacy, and informed decision-making, where individuals no longer feel isolated by their condition.
The Role of Education in Gene Therapy Awareness
Awareness and education surrounding gene therapies for hemophilia are critical to ensuring patient access and acceptance. Tragically, many patients remain unaware of the advances made in gene therapy, such as the development and efficacy of Hemgenix. It is essential for healthcare providers to take proactive roles in disseminating information about these treatments, explaining not only how they work but also their potential benefits and risks. With an understanding of modern treatments, patients are more likely to embrace therapy options that can significantly enhance their quality of life.
Furthermore, integrating education into patient care pathways can help individuals make informed decisions based on their unique health circumstances. As gene therapies continue to gain traction, the medical community must maintain open lines of communication about available treatment options and facilitate conversations about personal concerns, preferences, and expectations regarding hemophilia management. Empowering patients through education not only fosters confidence but helps create a future where living with hemophilia may be significantly less burdensome.
Frequently Asked Questions
What is hemophilia gene therapy and how does it work?
Hemophilia gene therapy, specifically Hemgenix treatment, aims to address the underlying cause of hemophilia B by delivering a corrected version of the gene responsible for producing clotting factor IX. This gene therapy involves using a modified virus to target the liver, where the clotting factor is produced, enabling the body to produce the factor itself, thereby reducing the need for frequent injections.
What are the latest advancements in gene therapy for hemophilia?
Recent advancements in gene therapy for hemophilia include the FDA approval of Hemgenix, a groundbreaking treatment for hemophilia B that allows for long-term production of clotting factor IX from a single infusion. Ongoing research continues to explore the impact of gene therapy on hemophilia management, making significant strides in reducing treatment burdens for patients.
How effective is Hemgenix for treating hemophilia B?
Hemgenix has shown promising results in clinical trials, with 94% of participants not requiring factor IX prophylaxis three years post-treatment. This highlights the potential for long-lasting effects and a significant improvement in the quality of life for individuals with hemophilia B.
What should patients expect during the hemophilia gene therapy treatment process?
During hemophilia gene therapy treatment, patients can expect to undergo a relatively routine infusion procedure. The treatment typically lasts about two hours, followed by a period of observation to monitor for side effects. Patients may also need to manage liver function post-treatment, which is closely monitored by their healthcare team.
What are the risks associated with hemsophilia gene therapy?
While hemophilia gene therapy, such as Hemgenix, presents significant benefits, there are potential risks including elevated liver enzymes and immune reactions to the modified virus. Patients are monitored closely during and after treatment to manage any adverse effects and ensure safety.
How does living with hemophilia change after gene therapy?
After undergoing gene therapy for hemophilia, many patients experience a dramatic decrease in the need for regular clotting factor injections. This shift not only improves physical health by reducing spontaneous bleeds but also enhances quality of life by alleviating the social and emotional burdens associated with frequent treatment.
What is the future of hemophilia B news and gene therapy treatments?
The future of hemophilia B news and gene therapy treatments looks promising, with ongoing research aimed at developing more effective therapies. As more gene therapies become available and existing treatments are refined, patients may have broader options that could further improve their care and quality of life.
| Key Point | Details |
|---|---|
| Introduction of Gene Therapy | Terence Blue became the first patient in New England to receive Hemgenix, a gene therapy for hemophilia B. |
| Living with Hemophilia | Terence has managed his hemophilia since infancy, requiring regular treatments and shots. |
| Advancements in Treatment | New synthetic factors and gene therapy promise significant improvements in managing hemophilia. |
| Market Challenges | High costs and limited patient interest pose challenges for gene therapy market viability. |
| Impact of Hemgenix | Blue’s factor IX levels increased from below 1% to 32% after receiving Hemgenix, indicating positive treatment effects. |
| Future Outlook | Long-term benefits of gene therapy are expected but cautious optimism remains among medical professionals. |
Summary
Hemophilia gene therapy represents a significant advancement in the treatment of hemophilia B. The recent success of Hemgenix has given patients like Terence Blue hope for a future with less reliance on frequent treatments, reflecting the potential for transformative healthcare solutions that gene therapies can provide. With ongoing research and the approval of additional therapies, the landscape of hemophilia treatment is evolving rapidly, promising improved quality of life and possibly even a cure for affected individuals.