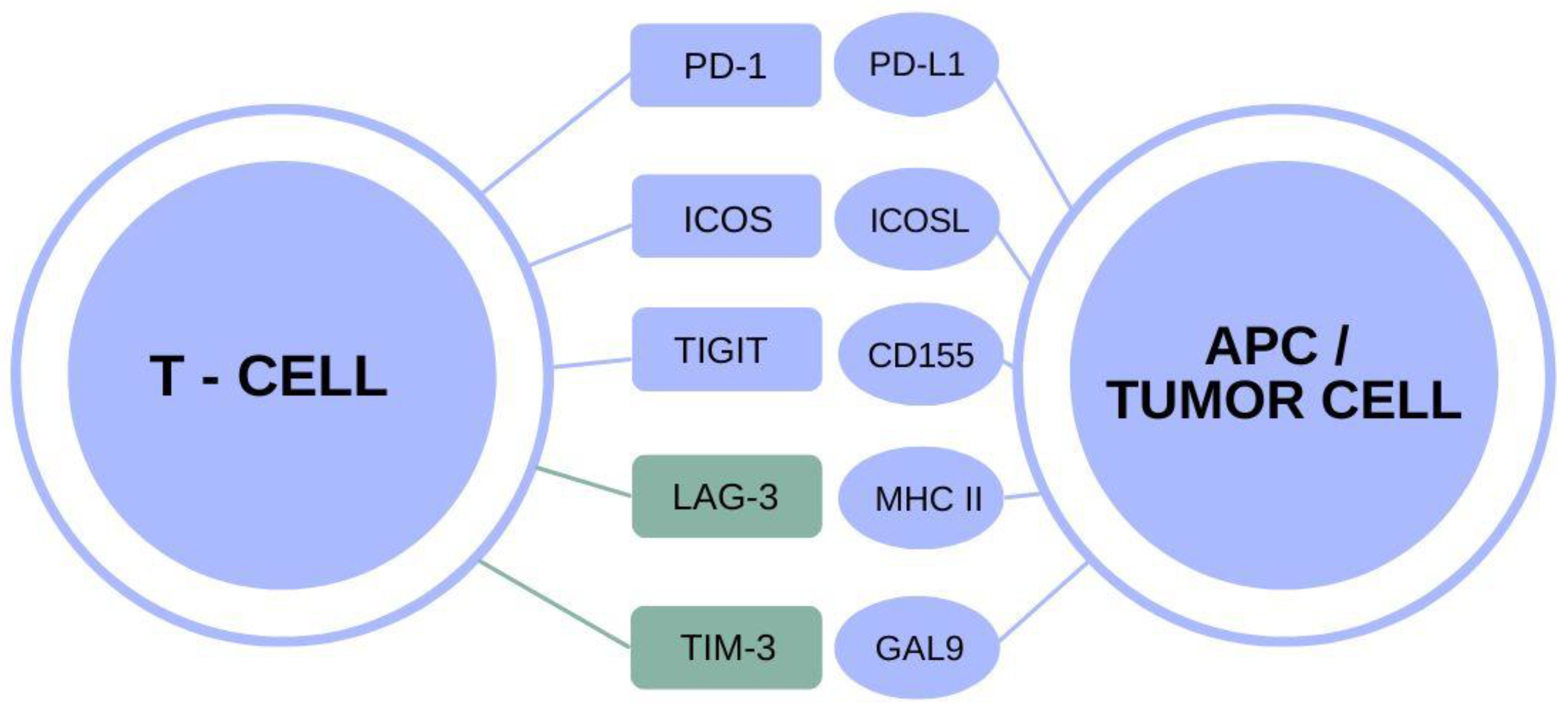
TIM-3 Alzheimer’s therapy represents a groundbreaking approach that leverages insights from cancer treatment to combat Alzheimer’s disease. Researchers have discovered that the TIM-3 molecule, a critical checkpoint in the immune system, plays a pivotal role in inhibiting microglia, the brain’s immune cells, from effectively clearing amyloid-beta plaques associated with Alzheimer’s. In recent studies, the deletion of the TIM-3 gene in mouse models demonstrated promising results, enhancing microglial function and subsequently improving cognitive performance. This innovative therapy could redefine Alzheimer’s disease treatment paradigms by utilizing anti-TIM-3 antibodies to block these inhibitory signals, thereby reinvigorating microglial activity. As we delve deeper into this exciting new frontier, the potential of TIM-3-based therapies shines brightly, offering hope for better outcomes in Alzheimer’s patients.
The innovative strategy involving TIM-3 therapy for Alzheimer’s disease is gaining traction as researchers explore its potential to revolutionize treatment paradigms. By focusing on the TIM-3 checkpoint molecule, scientists aim to reactivate the immune response within the brain, encouraging the clearance of toxic plaques that impair memory and cognition. This approach not only highlights the interplay between the immune system and neurodegenerative disorders but also illustrates how concepts from immune system cancer therapy can be repurposed to target Alzheimer’s pathology. With the role of microglia in plaque clearance now under the microscope, the research is paving the way for new interventions that may fundamentally alter the course of this devastating condition. As we understand more about the TIM-3 molecule’s functions and mechanisms, the promise of effective Alzheimer’s treatments could be on the horizon.
Understanding TIM-3 in Alzheimer’s Disease
The TIM-3 molecule has emerged as a critical factor in Alzheimer’s disease, particularly in the context of late-onset cases, which constitute around 90 to 95 percent of all Alzheimer’s diagnoses. A recent study highlighted the genetic link between TIM-3 and Alzheimer’s, suggesting that variations in the HAVCR2 gene, which codes for TIM-3, are associated with increased risk. This relationship underscores the importance of TIM-3 not just in immune regulation but also in the pathology of Alzheimer’s, revealing a potential target for therapeutic intervention.
In the brain, microglia—the primary immune cells—exhibit heightened expression of TIM-3 in the presence of amyloid-beta plaques. The inhibition caused by TIM-3 prevents microglia from performing their critical role in clearing these harmful plaques, which contribute to neurodegeneration. This impaired function highlights a dual role of TIM-3; while it serves to regulate immune response, its overexpression in Alzheimer’s patients can be detrimental, necessitating a deeper investigation into TIM-3 as a therapeutic target.
The Role of Microglia in Alzheimer’s Pathology
Microglia are often referred to as the immune soldiers of the brain, playing a crucial role in maintaining brain health and homeostasis. They not only support synaptic pruning during development to enhance memory retention but also respond to neurodegenerative changes. However, in Alzheimer’s disease, the activity of microglia is compromised due to the increased expression of TIM-3, which leads these cells to adopt a homeostatic state where they fail to engage and clear amyloid plaques effectively.
Research indicates that as Alzheimer’s progresses, the inability of microglia to remove plaques exacerbates cognitive decline. The selective expression of TIM-3 in activated microglia suggests a regulatory checkpoint that needs to be manipulated for therapeutic purposes. By targeting this pathway, we can potentially restore the phagocytic capacity of microglia, enhancing their ability to clear toxic debris and ultimately improving cognitive function in Alzheimer’s patients.
Exploring Anti-TIM-3 Antibodies for Alzheimer’s Treatment
The innovative application of anti-TIM-3 antibodies represents a promising frontier in Alzheimer’s therapy. These antibodies could block the inhibitory effects of TIM-3, empowering microglia to regain their plaque-clearing capabilities. Unlike traditional anti-amyloid therapies, which have failed to produce consistent results due to vascular complications, anti-TIM-3 therapy could offer a more targeted approach by enhancing the innate immune response within the brain.
Moreover, the potential for repurposing existing anti-TIM-3 antibodies that have been effective in cancer immunotherapy could expedite the transition to clinical trials for Alzheimer’s treatment. By focusing on the immune modulation via TIM-3 inhibition, researchers hope to create a dual impact: reducing pathological plaque accumulation while simultaneously fostering a healthier cognitive environment in the aging brain.
Gene Editing Techniques in Alzheimer’s Research
Gene editing plays a pivotal role in advancing our understanding of Alzheimer’s disease, particularly in studying the effects of TIM-3 on neurodegeneration. The use of genetically edited mouse models, specifically those with the HAVCR2 gene deletion, has provided vital insights into how TIM-3 influences microglial function and plaque interaction. These models facilitate real-time observation of cognitive behavior and memory retention, which are critical in assessing the efficacy of new therapies.
By adjusting genetic markers and pathways associated with TIM-3, researchers can elucidate the molecular mechanisms at play in Alzheimer’s pathology. This approach not only helps pinpoint the exact role of TIM-3 in disease but also paves the way for evaluating potential gene-based therapies and small-molecule interventions aimed at disrupting inhibitory checkpoints in the immune response.
Implications of Checkpoint Molecules in Neurodegenerative Diseases
Checkpoint molecules, such as TIM-3, illustrate a fascinating intersection between immunology and neurodegenerative disease. Originally studied in cancer therapy, these molecules regulate the immune response, preventing over-activation that could lead to autoimmunity. However, their role in the context of Alzheimer’s reveals a paradox: while they are essential for normal immune function, their dysregulation can contribute to the pathogenesis of neurodegenerative diseases.
Utilizing checkpoint inhibition strategies could revolutionize treatment paradigms for Alzheimer’s disease, allowing the immune system to effectively engage toxic plaques rather than leaving them unchecked. Continued research into checkpoint molecule dynamics may uncover novel strategies that not only enhance memory clearance but also provide cognitive support to patients as the disease progresses.
Cognitive Behavior Assessment in Alzheimer’s Research
In Alzheimer’s research, the assessment of cognitive behavior, particularly in experimental models, is crucial for understanding the impact of therapies targeting the TIM-3 molecule. Standard tests, such as maze navigation and memory retention tasks, gauge the extent of cognitive impairment associated with plaque accumulation. These assessments are vital for correlating microglial activity with behavioral outcomes, thus providing a comprehensive picture of therapeutic effectiveness.
The ability of modified mice lacking TIM-3 to regain cognitive function when plaques are cleared highlights the potential for effective interventions. Such behavioral improvements not only indicate successful plaque management but also translate to meaningful changes in the quality of life for patients. Understanding these behavioral metrics will be integral in shaping future clinical trials aimed at developing TIM-3 targeted therapies.
Future Directions in Alzheimer’s Research
With the insights gained from TIM-3 research, the path forward for Alzheimer’s treatment is becoming more defined. The exploration of anti-TIM-3 antibodies opens new avenues for clinical trials, as the therapeutic applicability of these agents expands beyond cancer to neurodegenerative conditions. By focusing on drugs that can enhance microglial function without causing adverse effects, researchers are hopeful for a breakthrough in Alzheimer’s treatment.
Furthermore, the ongoing collaboration between immunologists and neurologists will be essential in translating these findings from bench to bedside. As large-scale studies begin to leverage genetically engineered models that closely resemble human neurodegenerative processes, the potential for discovering effective therapies increases, bringing hope for Alzheimer’s patients and their families.
Clinical Challenges and Opportunities
Despite recent advances, the road to effective Alzheimer’s therapies remains fraught with challenges. The complexity of the disease, coupled with the multifaceted roles of molecules like TIM-3, necessitates a comprehensive understanding of both immune and neural interactions. Clinical trials focusing on TIM-3 modulation must address potential off-target effects and ensure that therapies do not inadvertently disrupt critical immune functions that are vital for maintaining overall brain health.
Nonetheless, the opportunities presented by TIM-3 targeting are vast. As researchers refine their approaches and gather more data on the efficacy of anti-TIM-3 therapies, the landscape of Alzheimer’s treatment could change significantly. Innovative strategies that integrate immune modulation with traditional therapeutic techniques could provide synergistic effects, ultimately leading to more effective interventions for Alzheimer’s disease.
The Importance of Long-Term Studies in Alzheimer’s Therapy
Long-term studies are crucial to fully understand the impact of TIM-3 inhibition on Alzheimer’s disease progression. Evaluating therapies over extended periods allows researchers to track not only cognitive improvement but also potential side effects and immune responses. Such data will be instrumental in determining the safety and efficacy of anti-TIM-3 antibodies as a standardized treatment option.
Participating in long-term studies will also help clarify how TIM-3 therapy interacts with the aging brain, contributing to our understanding of neurodegeneration over time. As we gather more evidence from both animal models and human trials, the knowledge accrued will inform future research directions and refine therapeutic strategies in combating Alzheimer’s disease.
Frequently Asked Questions
What is TIM-3 Alzheimer’s therapy and how does it work?
TIM-3 Alzheimer’s therapy targets the TIM-3 molecule, which is linked to late-onset Alzheimer’s disease. By inhibiting TIM-3, microglia, the brain’s immune cells, are freed from restraining mechanisms, allowing them to clear amyloid plaques that accumulate in Alzheimer’s patients, thus potentially improving memory and cognitive function.
How does TIM-3 relate to the immune system and Alzheimer’s disease treatment?
In Alzheimer’s disease treatment, TIM-3 functions as a checkpoint molecule that inhibits microglial activity. This inhibition prevents microglia from effectively clearing harmful amyloid plaques in the brain. Therapies that block TIM-3, like anti-TIM-3 antibodies, aim to enhance microglial activity and restore their ability to combat Alzheimer’s-related plaques.
What role do microglia play in TIM-3 Alzheimer’s therapy?
Microglia, the immune cells of the brain, are critical in TIM-3 Alzheimer’s therapy. Normally, TIM-3 suppresses microglial functions, preventing them from clearing amyloid plaques. By targeting TIM-3, therapies can enable microglia to engulf and remove these plaques, which may help alleviate symptoms of Alzheimer’s disease.
Are anti-TIM-3 antibodies effective in treating Alzheimer’s disease?
Yes, anti-TIM-3 antibodies show promise in treating Alzheimer’s disease by blocking the inhibitory effects of the TIM-3 molecule on microglia. This allows for enhanced clearance of amyloid plaques and potential improvements in cognitive function, as seen in studies with mice.
What is the significance of TIM-3 in late-onset Alzheimer’s disease?
TIM-3 is significant in late-onset Alzheimer’s disease as it has been identified as a genetic risk factor. Its expression on microglia is significantly higher in Alzheimer’s patients, leading to impaired plaque clearance and cognitive decline. Research suggests that targeting TIM-3 could be a potential therapeutic strategy.
What are the potential outcomes of TIM-3 Alzheimer’s therapies in humans?
The potential outcomes of TIM-3 Alzheimer’s therapies in humans include improved cognitive function and memory retention through enhanced microglial activity and plaque clearance. Early studies on mice have shown promise, paving the way for human applications of anti-TIM-3 therapies.
How does TIM-3 Alzheimer’s therapy differ from traditional Alzheimer’s treatments?
TIM-3 Alzheimer’s therapy differs from traditional treatments by focusing on modulating the immune response through checkpoint inhibition rather than solely targeting amyloid beta accumulation. This innovative approach aims to enhance the natural capabilities of microglia to clear plaques, potentially leading to better clinical outcomes.
What research supports the efficacy of TIM-3 Alzheimer’s therapy?
Research supporting TIM-3 Alzheimer’s therapy includes studies demonstrating that genetically altering mice to delete the TIM-3 gene leads to improved plaque clearance and cognitive function. Findings indicate that inhibiting TIM-3 allows microglia to better respond to amyloid plaques, suggesting a novel pathway for Alzheimer’s treatment.
Can TIM-3 Alzheimer’s therapy be combined with other treatments?
Yes, TIM-3 Alzheimer’s therapy can potentially be combined with other treatments, such as traditional anti-amyloid therapies, to enhance overall therapeutic efficacy. The targeted nature of TIM-3 inhibition may improve patient outcomes when used alongside existing Alzheimer’s treatments.
What are the next steps in TIM-3 Alzheimer’s therapy research?
The next steps in TIM-3 Alzheimer’s therapy research involve testing the efficacy of human anti-TIM-3 antibodies in Alzheimer’s mouse models that simulate human brain responses. Ongoing studies aim to confirm the potential of TIM-3 inhibition to halt plaque development and improve cognitive function in humans.
| Key Points |
|---|
| Alzheimer’s and Immune System Link |
| The immune system checkpoint molecule TIM-3 is an inhibitory factor in Alzheimer’s by preventing microglia from attacking amyloid plaques. |
| Research Findings |
| Deleting TIM-3 from microglia in mice led to reduced amyloid plaque accumulation and improved memory function in tests. |
| Alzheimer’s Demographics |
| 90-95% of Alzheimer’s cases are late-onset, associated with genetic polymorphisms in the TIM-3 gene. |
| Therapeutic Potential |
| Anti-TIM-3 therapy may be a promising approach for Alzheimer’s treatment by freeing microglia to clear plaques. |
| Research Collaboration |
| The study was a collaborative effort at Harvard involving multiple researchers over five years. |
Summary
TIM-3 Alzheimer’s therapy represents a promising new approach in the battle against Alzheimer’s disease. By targeting the TIM-3 checkpoint molecule, researchers have observed improved cognitive function and plaque reduction in mouse models. This innovative method, which has potentially repurposable applications from cancer treatment, could provide a significant advancement in therapeutic strategies aimed at combating the neurodegenerative effects characterizing Alzheimer’s disease.