Patient Safety in Medical Research: Funding Cuts Impact
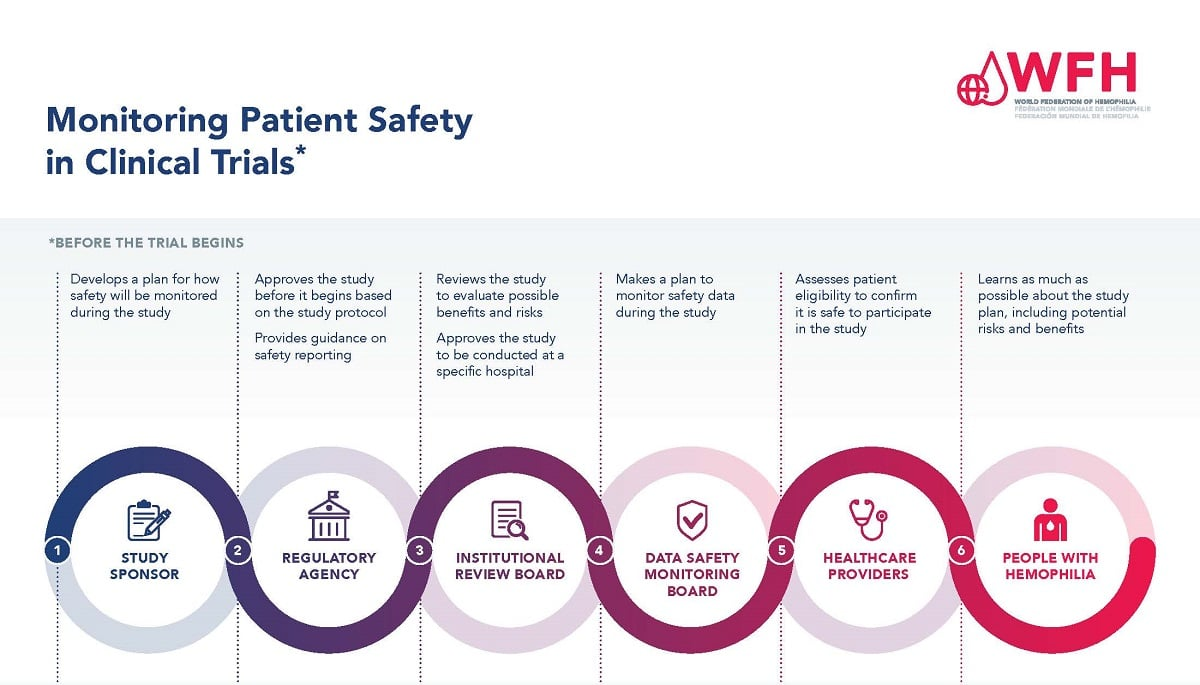
Patient safety in medical research is a critical aspect that underpins the ethical conduct of studies involving human participants.The integrity of clinical trials relies heavily on robust oversight mechanisms, such as Institutional Review Board (IRB) oversight, which ensure that patient rights in research are respected and upheld.
Medical Research Ethics: Funding Cuts and Patient Safety
Medical research ethics is a cornerstone of safeguarding human participants in clinical studies, ensuring that their rights and safety are prioritized throughout the research process.As investigations evolve, the rigorous oversight by Institutional Review Boards (IRBs) becomes indispensable, particularly in light of recent disruptions in medical research funding.