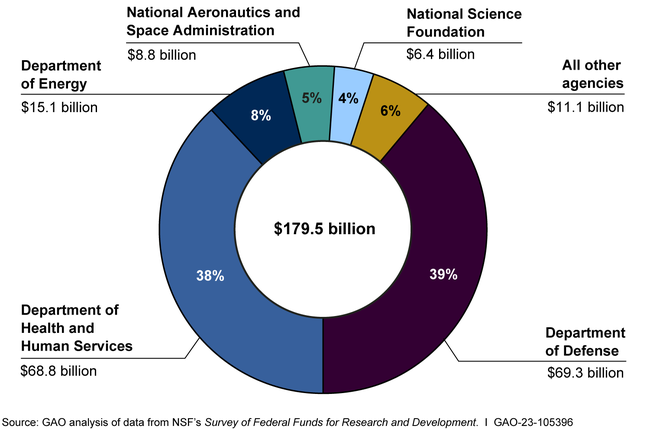
Medical research ethics is a cornerstone of safeguarding human participants in clinical studies, ensuring that their rights and safety are prioritized throughout the research process. As investigations evolve, the rigorous oversight by Institutional Review Boards (IRBs) becomes indispensable, particularly in light of recent disruptions in medical research funding. The halt in funding not only impacts the ethical governance of studies but also raises significant concerns about clinical trial safety and safeguarding patient rights in research. Moreover, it is essential for researchers to navigate the complexities of NIH research grants, which provide crucial financial resources while adhering to stringent ethical guidelines. Hence, maintaining a robust framework for medical research ethics is vital for fostering trust and innovation in healthcare.
The principles of ethics in medical studies focus on the protection of participants, ensuring that the dignity and autonomy of individuals are upheld during research initiatives. Oversight mechanisms, like Institutional Review Boards, play a pivotal role in monitoring the ethical aspects of research, guiding the collaboration between universities and healthcare institutions. In an era where funding for medical investigations is at risk, maintaining rigorous standards of patient care is more crucial than ever. This intersection of ethical responsibility and financial support defines the landscape of human research and its potential to drive advancements in medicine. By prioritizing ethical practices, researchers can assure participants that their well-being is paramount, helping to restore faith in the research process.
The Impact of Funding Cuts on Medical Research Safety
The recent freeze of over $2 billion in federal research grants to Harvard has far-reaching consequences for the safety of patients involved in medical research. As institutions like Harvard rely on these funds to support their Institutional Review Boards (IRBs) and research ethics programs, the halt in funding restricts their ability to enforce vital oversight mechanisms. Without these resources, studies may be improperly monitored, putting the health and well-being of participants at risk. This situation underscores the critical relationship between adequate funding and the efficacy of patient safety protocols in clinical trials.
Furthermore, the implications of funding cuts extend beyond immediate patient safety concerns. They also threaten the comprehensive training and support provided to researchers and institutional review boards. A well-funded IRB system not only reviews new study proposals to ensure ethical compliance but also offers continuing education on ethical standards and regulatory changes for researchers. When financial resources dwindle, the capacity for ongoing education diminishes, allowing gaps in knowledge that could ultimately compromise study integrity and patient rights in research.
Understanding IRB Oversight in Medical Research
Institutional Review Boards (IRBs) are pivotal in safeguarding the welfare of participants in medical research studies. These entities conduct thorough reviews of research protocols to verify that studies adhere to ethical guidelines, including informed consent and risk assessment. IRBs help mitigate potential harms by evaluating the risk-benefit ratio of proposed medical trials, ensuring that the rights of all involved—especially vulnerable populations—are prioritized. The role of IRBs has become even more pronounced with the implementation of the NIH’s single IRB policy for multisite studies, which streamlines oversight and regulation.
Moreover, IRBs are instrumental in maintaining public trust in the research process. By upholding patient rights in research and advocating for participant welfare, IRBs serve as a vital checks-and-balances system in clinical research. They work diligently to manage the ethical complexities inherent in scientific studies, particularly those involving human subjects. As oversight mechanisms face additional funding challenges, it remains crucial that IRBs continue to operate effectively to uphold the integrity and safety of clinical trials.
Patient Rights in Medical Research: A Fundamental Aspect
Patient rights are a cornerstone of ethical medical research. This includes the right to informed consent, where participants must be fully educated on the nature of the study, its risks, and their rights. The ethical responsibility to respect these rights falls heavily on research institutions and their IRBs, whose involvement ensures that patient autonomy is maintained throughout the research process. When funding cuts hamper the capabilities of these boards, the risk arises that participant rights may not be sufficiently protected, leading to potential ethical violations.
Furthermore, the protection of patient rights also influences public trust in medical research. When participants feel secure in their rights and safeguards, they are more likely to engage in studies, which is essential for advancing scientific knowledge. Conversely, funding limitations that result in less oversight can instigate skepticism among potential contributors to research, further complicating efforts to secure diverse participant pools necessary for robust study findings. Protecting patient rights is not just a regulatory issue; it is essential for maintaining the integrity of the entire research enterprise.
How NIH Research Grants Protect Patients
NIH research grants play a crucial role in ensuring that studies involving human participants are conducted ethically and safely. Funds provided by the NIH are not simply sources of finance; they also underpin the mechanisms—such as IRBs—that are essential for reviewing protocols and ensuring compliance with ethical standards. By prioritizing research that meets rigorous scrutiny, NIH grants enhance the overall safety and legal compliance of clinical trials, thereby protecting the interests and rights of participants.
Additionally, NIH-funded studies typically follow strict guidelines outlined in their grant regulations. These include mandatory reviews and adherence to local, state, and federal regulations, confirming that ethical practices are embedded in the research lifecycle. In this way, NIH funding acts as a safeguard, ensuring that research not only contributes to scientific advancement but also prioritizes the welfare of those who volunteer for studies, ultimately fostering a culture of responsibility and integrity within the research community.
The Role of Clinical Trial Safety in Medical Advancements
Clinical trial safety is paramount for the success of medical research and innovation. Ensuring patient safety throughout the trial duration is not only an ethical obligation but also directly influences the credibility of the research findings. Meticulous safety protocols, overseen by IRBs, help identify and address potential risks, allowing researchers to take proactive measures to protect participants while enabling the advancement of crucial medical knowledge.
Moreover, transparent reporting and vigilant monitoring of adverse events during clinical trials can significantly enhance public confidence in medical research. When participants see that their safety is prioritized, they are more likely to participate in future studies. Thus, fostering a robust framework around clinical trial safety ultimately contributes to better patient recruitment, accelerated research timelines, and the development of novel therapies that benefit society.
The Historical Context of Research Ethics
The evolution of medical research ethics is deeply rooted in historical injustices. Notable studies, such as the Tuskegee syphilis study, have revealed the catastrophic consequences of inadequate ethical oversight and disregard for patient welfare. These incidents instigated a paradigm shift that led to the establishment of stringent regulations and ethical frameworks, including the establishment of IRBs to monitor research practices comprehensively. Understanding this history is crucial in ensuring that medicine learns from past mistakes to prevent similar violations from occurring in modern research.
This historical awareness compels the research community to remain vigilant and committed to upholding ethical standards. The lessons learned from past transgressions continue to influence the design and implementation of ethical guidelines in contemporary research settings, especially regarding patient rights and consent. By acknowledging the mistakes of the past, researchers can better safeguard against them, promoting a culture of respect and ethical integrity in all future medical advancements.
Navigating the Challenges of Multisite Collaborative Research
Conducting multisite collaborative research presents unique challenges, particularly in ensuring consistent ethical oversight across various institutions. The SMART IRB system aims to streamline this process by allowing a single IRB to oversee multiple sites engaged in a study. However, without robust funding and support, these collaborative efforts may face delays and complications that hinder the progress of vital research initiatives and compromise patient safety.
Additionally, funding cuts not only affect the operational aspects of the SMART IRB system but also the buy-in from participating institutions. When financial resources are strained, institutions may be reluctant to engage fully with collaborative research efforts, fearing the consequences of failing to meet ethical and regulatory requirements. Thus, it is crucial to ensure adequate funding is available to support these vital functions, which ultimately enhances the safety and welfare of participants involved in multisite research.
The Significance of Collaboration in Research Ethics
Collaboration is essential in medical research, particularly when addressing complex health issues that transcend single institutions. Effective collaboration fosters the sharing of knowledge, resources, and ethical best practices among researchers, IRBs, and regulatory bodies. It leads to improved oversight mechanisms and enhances the collective understanding of how to protect participant rights while advancing scientific inquiry.
Moreover, collaborative efforts in research can lead to innovative solutions and faster advancements in medical treatments. By bringing together diverse perspectives and expertise, collaborative research fosters an environment of shared accountability where ethical considerations become a collective priority, reinforcing the integrity of medical research. Ensuring that these collaborative networks remain funded and supported is critical to maintaining the standards of ethical research practices.
Future Directions in Medical Research Ethics
As the landscape of medical research continues to evolve, the need for robust ethical oversight becomes increasingly important. Future directions in medical research ethics will rely on adapting to new technologies, methodologies, and societal expectations. For instance, the growth of digital health tools and data-sharing – while promising significant advancements – also raises ethical challenges that must be addressed through comprehensive regulatory frameworks.
Ensuring effective ethical oversight in this evolving landscape will require ongoing commitment from research institutions, funding bodies, and ethical review boards. Investing in training for researchers and IRBs to understand and navigate emerging ethical challenges is essential. By prioritizing ethics and safeguarding patient rights in future medical research, we can build public trust and foster a research environment that prioritizes health and safety.
Frequently Asked Questions
What is the significance of IRB oversight in medical research ethics?
IRB oversight is crucial in medical research ethics as it ensures the protection of human participants. Institutional Review Boards (IRBs) review and approve research protocols, guaranteeing compliance with ethical standards, laws, and regulations. This structured oversight mitigates potential risks to patient rights and safety during clinical trials.
How do NIH research grants contribute to patient rights in medical research?
NIH research grants play an important role in safeguarding patient rights in medical research by funding studies that are subject to rigorous ethical review. This includes the requirement for all proposed human subject research to undergo IRB oversight, ensuring informed consent and the protection of participant welfare throughout the research process.
What impact does clinical trial safety have on medical research ethics?
Clinical trial safety is foundational to medical research ethics as it prioritizes the health and well-being of research participants. Ethical guidelines mandate thorough risk assessment and ongoing monitoring of clinical trials, ensuring that participant safety is maintained and that any adverse events are promptly addressed.
Why is funding essential for maintaining patient rights in research?
Funding is vital for maintaining patient rights in research because it supports the infrastructure for IRB oversight and ethical compliance. Adequate funding enables institutions to properly review and monitor studies, ensuring that patient rights and safety in medical research are not compromised by resource limitations.
What are the consequences of halting medical research funding on clinical trial safety?
Halting medical research funding can jeopardize clinical trial safety by interrupting ongoing studies and limiting the ability to ensure proper oversight. This can lead to increased risks for participants, erosion of trust in the research process, and potential harm to patient communities due to suspended research efforts.
How does the SMART IRB system enhance medical research ethics?
The SMART IRB system enhances medical research ethics by facilitating multi-site study reviews through a single IRB. This streamlined approach reduces delays and bureaucratic hurdles while ensuring comprehensive ethical oversight, thus upholding patient rights and safety across multiple research sites.
What role does medical research funding play in ethical oversight?
Medical research funding is essential for ethical oversight as it supports the operations of IRBs and the implementation of standard ethical practices. Without sufficient funding, institutions may struggle to maintain the necessary review processes that protect participants and uphold the integrity of medical research.
In what ways do historical events shape current medical research ethics?
Historical events, such as unethical medical experiments, have significantly influenced the development of medical research ethics. These events highlight the necessity for stringent oversight, leading to the establishment of IRBs and regulatory frameworks that prioritize participant safety, informed consent, and ethical conduct in research.
| Key Points | Details |
|---|---|
| Impact of Funding Cuts | The freeze of over $2 billion in federal research grants disrupts research efforts and undermines patient safety. |
| Importance of IRBs | Institutional Review Boards review and oversee research ensuring participant rights and safety. |
| Role of SMART IRB | Facilitates oversight of multisite research studies, enhancing efficiency in collaboration. |
| Historical Context | Past abuses in medical research underscore the need for rigorous ethical oversight. |
| Effects on Ongoing Research | Funding cuts halt studies, prevent site expansions, and delay vital research tasks. |
Summary
Medical research ethics are crucial to ensuring the protection of participants involved in clinical studies. The recent halt in funding has significant implications for patient safety and the integrity of the research process. As institutional review boards (IRBs) play an essential role in safeguarding the rights and welfare of research participants, any disruption in their functioning compromises the ethical framework established to prevent past abuses in medical experimentation. Therefore, continued support and funding for ethical oversight in medical research are imperative for maintaining public trust, advancing scientific collaboration, and ultimately enhancing patient safety.