Patient safety in medical research is a critical aspect that underpins the ethical conduct of studies involving human participants. The integrity of clinical trials relies heavily on robust oversight mechanisms, such as Institutional Review Board (IRB) oversight, which ensure that patient rights in research are respected and upheld. However, recent halts in medical research funding threaten to undermine these safeguards, potentially compromising the ethical foundation necessary for safe and effective research practices. As institutions face significant disruptions, it becomes imperative to recognize the vital role that adequate funding plays in maintaining research ethics and ensuring patient welfare. Ultimately, the long-term effects on research innovation and patient trust in the healthcare system will hinge on our collective commitment to prioritize patient safety in medical research.
Ensuring the well-being of individuals in scientific studies is paramount, particularly in the realm of human subjects research. The oversight provided by bodies such as IRBs is instrumental in safeguarding participant interests, thereby fostering ethical standards within the field. Disruptions in funding can severely impact the systems designed to protect participants, raising concerns about the future of research ethics and overall patient safety. When we consider clinical studies and academic investigations, we must advocate for the necessary resources that allow these protective frameworks to function effectively. With the interconnectedness of funding, innovation, and trust, the landscape of medical exploration relies on an unwavering dedication to participant welfare.
The Critical Role of Patient Safety in Medical Research
Patient safety is paramount in medical research, especially when human subjects are involved. Researchers are obligated to ensure that participants are not only informed but also protected from potential risks associated with clinical trials. Institutional Review Boards (IRBs) play a vital role in this process, carefully evaluating every research proposal to ensure the ethical treatment of participants. The halt in funding directly impacts the ability to maintain rigorous oversight, thereby jeopardizing the fundamental principle of patient safety in research.
As patient safety in medical research is scrutinized, the integrity of IRB oversight becomes even more essential. IRBs are tasked with examining the scientific validity and ethical considerations of research proposals, ensuring that patient rights are not compromised. With funding cuts, these boards may struggle to uphold their responsibilities, leading to potential lapses in protecting vulnerable populations. The trust that patients place in researchers hinges on the effectiveness of these protections, which are now at risk due to financial constraints.
Medical Research Funding and Its Implications
Medical research funding is the foundation upon which successful clinical trials are built. Without adequate financial support, the ability to conduct comprehensive studies ceases, limiting the advancements in medical treatments and technologies. The recent freeze on more than $2 billion in federal research grants has significant ramifications beyond just funding; it affects the collaborative infrastructure necessary for ethical research practices such as those governed by IRBs.
Funding is not just about dollars; it represents the commitment to uphold research ethics and patient safety. Cuts in medical research funding impede the progress of essential studies and can result in the discontinuation of research initiatives that protect patient rights. As budget allocations shrink, many institutions may be forced to prioritize which studies to pursue, potentially overlooking vital research necessary for patient welfare and public health advancements.
The Impact of IRB Oversight on Clinical Trials
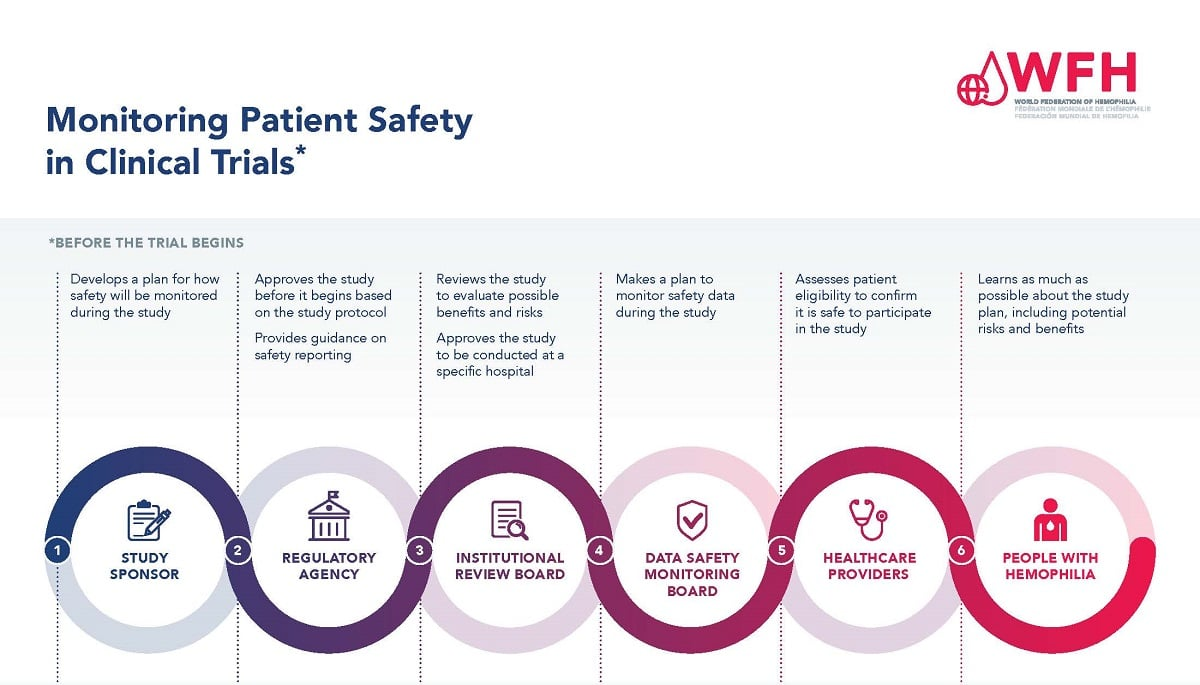
IRB oversight serves as a cornerstone of clinical trials, ensuring that ethical standards are met while reducing risks to participants. These boards assess various aspects of research proposals, including informed consent and risk identification, helping to safeguard participant rights and safety. The current funding freeze jeopardizes the capability of IRBs to perform these functions effectively, which may lead to insufficient safeguarding of participants in clinical studies.
With IRBs facing resource constraints due to funding cuts, the thorough review process for clinical trials could diminish. This can result in less rigorous assessments, potentially endangering participants’ safety and diluting the ethical standards established over decades. By threatening IRB oversight, the halt in funding not only stalls research progress but also undermines public confidence in clinical trials, which can have long-lasting effects on patient recruitment and retention.
Ensuring Patient Rights in Research Amid Funding Cuts
Patient rights in research are designed to protect individuals from exploitation and ensure informed consent is obtained before participation. The challenges posed by recent funding cuts risk undermining these rights, as many institutions may lack the resources to fulfill essential ethical obligations. This could pave the way for ethical breaches, where participants are inadequately informed of risks or coerced into participation due to a lack of alternatives.
Furthermore, the financial constraints may lead to an erosion of transparency in research practices. Institutions may become more focused on completing studies to recoup costs rather than ensuring ethical compliance and safeguarding patient rights. With diminished oversight, researchers may find themselves in situations where ethical decision-making becomes compromised, lessening the protection framework that has been established for research participants over time.
Research Ethics and the Future of Medical Studies
Research ethics encompasses the moral principles guiding researchers in the conduct of studies involving human subjects. The ongoing funding challenges threaten to divert attention from these ethics, as institutions grapple with financial uncertainties. When funding is no longer guaranteed, the commitment to maintaining high ethical standards may falter, impacting the quality and integrity of medical research.
As researchers struggle to navigate a landscape influenced by budget cuts, the fundamental tenets of research ethics must remain at the forefront of discussions. Institutions need to advocate for a return to stable funding to preserve the ethical oversight provided by IRBs and ensure that all research activity prioritizes patient safety and well-being. The future of medical studies depends on restoring the balance between financial support and unwavering adherence to ethical practices.
The Historical Context of Research Oversight
Understanding the historical context of research oversight is crucial in recognizing the necessity of patient safety mechanisms. Many research scandals throughout history have led to significant changes in ethical regulations, aiming to protect human subjects from harm. The legacy of these historical events underscores the importance of robust IRB processes and adequate funding to ensure compliance with ethical standards in present-day research.
Additionally, history provides valuable lessons about the consequences of ethical breaches in research. The establishment of stringent oversight frameworks has stemmed from past injustices, emphasizing the continual need to protect patients in medical trials. In light of the current funding cuts, recalling these pivotal moments reminds us of the importance of investing in the protection of human subjects to prevent history from repeating itself.
Navigating Current Challenges in Collaborative Research
Collaborative research, often vital for advancements in medical science, relies on effective coordination among institutions, supported by adequate funding and a streamlined IRB approval process. The recent funding cuts present significant barriers to collaboration, as host institutions struggle to absorb the financial and operational repercussions of halted projects. This has created a backlog of pending studies, limiting access for potential participants and threatening the advancement of critical medical research.
The challenges arising from insufficient funding also extend to the ability of research teams to communicate effectively and share results across various sites. When collaborative networks are disrupted, the safety and rights of research participants may be compromised. A robust collaborative framework ensures that ethics are maintained across all participating institutions, reaffirming the importance of acquiring dedicated resources to sustain ongoing efforts in medical research.
Community Engagement and Trust in Research
Community engagement is essential for developing trust in research, particularly when human subjects are involved. Researchers must prioritize building relationships with community members, ensuring that they understand their rights and the purpose of the studies they may be invited to join. As funding cuts threaten the capacity to maintain these relationships, the risk of alienating communities and diminishing trust in research becomes increasingly prevalent.
Trust in medical research is foundational for recruiting participants and securing informed consent. When funding hampers outreach efforts, communities may become skeptical of the intent behind research initiatives, fearing exploitation rather than collaboration. Researchers must navigate these challenges by reaffirming their commitment to ethical practices and patient safety, emphasizing the role of community collaboration in achieving successful research outcomes.
The Future of Patient Safety Strategies in Research
As the landscape of medical research changes, the future of patient safety strategies must evolve accordingly. Continuous education, advocacy, and the implementation of ethical standards are imperative to protect research subjects amid emerging challenges. Partnerships between institutions and regulatory bodies must be fortified to ensure that the principles of patient safety guide research driven by innovative funding models.
In conclusion, the importance of focusing on patient safety in research cannot be overstated. As financial resources fluctuate, the commitment to uphold ethical standards and protect individuals participating in clinical trials must remain unwavering. The future of medical research hinges on collaborative efforts to develop sustainable funding solutions that prioritize patient rights and enhance the integrity of IRB oversight.
Frequently Asked Questions
What is patient safety in medical research and why is it important?
Patient safety in medical research refers to the measures and practices aimed at protecting the health, rights, and welfare of individuals participating in clinical trials and studies. It is paramount because it ensures that researchers adhere to high ethical standards and regulatory requirements, ultimately safeguarding participants from potential harm. This concept is closely tied to research ethics and IRB oversight, ensuring that all studies prioritize participant safety and informed consent.
How does IRB oversight enhance patient safety in medical research?
IRB oversight enhances patient safety in medical research by rigorously reviewing study protocols, ensuring that research complies with ethical guidelines and legal requirements. Institutional Review Boards (IRBs) evaluate risks and benefits for participants, establish informed consent processes, and monitor ongoing trials to safeguard participant rights. This oversight is critical for maintaining trust in clinical trials, thereby promoting patient safety and the integrity of the research.
What impact does medical research funding have on patient safety?
Medical research funding plays a vital role in maintaining patient safety by enabling thorough oversight and compliance with ethical standards. Adequate funding supports necessary IRB evaluations, training for researchers on participant rights, and the development of robust safety protocols. When funding is cut, as seen with recent federal research grants, the quality and oversight of medical research can diminish, ultimately jeopardizing the safety and rights of patients involved in clinical trials.
How can patients ensure their rights are protected in medical research?
Patients can ensure their rights are protected in medical research by being informed about the study protocols, potential risks, and benefits before agreeing to participate. Engaging with IRBs or asking questions about the consent process, study aims, and safety measures are crucial steps. Furthermore, being aware of existing patient rights in research, such as the right to withdraw at any time, empowers participants to safeguard their well-being during clinical trials.
What are the historical factors that have shaped patient safety in medical research?
Historical events, such as unethical studies like the Tuskegee syphilis study and WWII medical experiments, have significantly shaped patient safety in medical research. These instances highlighted the need for stringent ethical oversight and the establishment of IRBs to protect participants from exploitation and harm. Such historical contexts underline the importance of transparency, informed consent, and vigilant monitoring in safeguarding patient rights and ensuring ethical conduct in research.
What challenges arise from funding cuts in medical research regarding patient safety?
Funding cuts in medical research pose severe challenges to patient safety by disrupting ongoing studies, limiting IRB oversight, and reducing resources for safety monitoring. Researchers may face increased delays in starting new trials or expanding existing ones, which could lead to compromised participant safety and diminished trust in the research process. These challenges can ultimately affect the quality and reliability of data gathered, hindering advancements in healthcare.
What role do clinical trials play in enhancing patient safety in research?
Clinical trials are crucial for enhancing patient safety in research as they provide structured frameworks where new therapies are tested for efficacy and safety before wider application. Rigorous protocols under the oversight of IRBs ensure participants receive adequate information and that their safety is monitored continuously throughout the trial. These trials help establish safe dosages and identify potential side effects, thereby prioritizing patient safety in the medical research landscape.
How does the SMART IRB initiative support patient safety in research?
The SMART IRB initiative supports patient safety in research by streamlining the review process for multisite studies, ensuring a single IRB oversees research across multiple locations. This coordinated approach enhances efficiency while maintaining rigorous oversight, ultimately safeguarding the rights and welfare of participants involved in clinical trials. By reducing administrative burdens, the SMART IRB facilitates timely studies that prioritize patient safety and ethical research practices.
| Key Point | Description |
|---|---|
| Funding Cuts | The halt of over $2 billion in federal research grants affects patient safety and research efforts. |
| SMART IRB | This system facilitates oversight of multi-site medical research but is at risk due to funding cuts. |
| Role of IRBs | Institutional Review Boards (IRBs) ensure compliance with regulations and protect patient rights in research studies. |
| Historical Context | Past unethical medical experiments led to the establishment of strict oversight and evaluation processes for research. |
| Impact on Participants | Interruptions in research pose risks to patient safety and may decrease public trust in medical studies. |
| Future of Research | Funding cuts may stifle innovation and collaboration necessary for advancing medical treatments. |
Summary
Patient safety in medical research is significantly compromised by the recent funding cuts affecting crucial oversight systems. The halt of federal grants has disrupted the collaborative framework established to protect participants involved in clinical studies, leading to potential risks and delays in ongoing research. This situation underscores the importance of maintaining robust funding and support for research oversight to ensure that the rights and safety of patients are prioritized in all medical investigations.